Research
Outline of Scientific Research Program
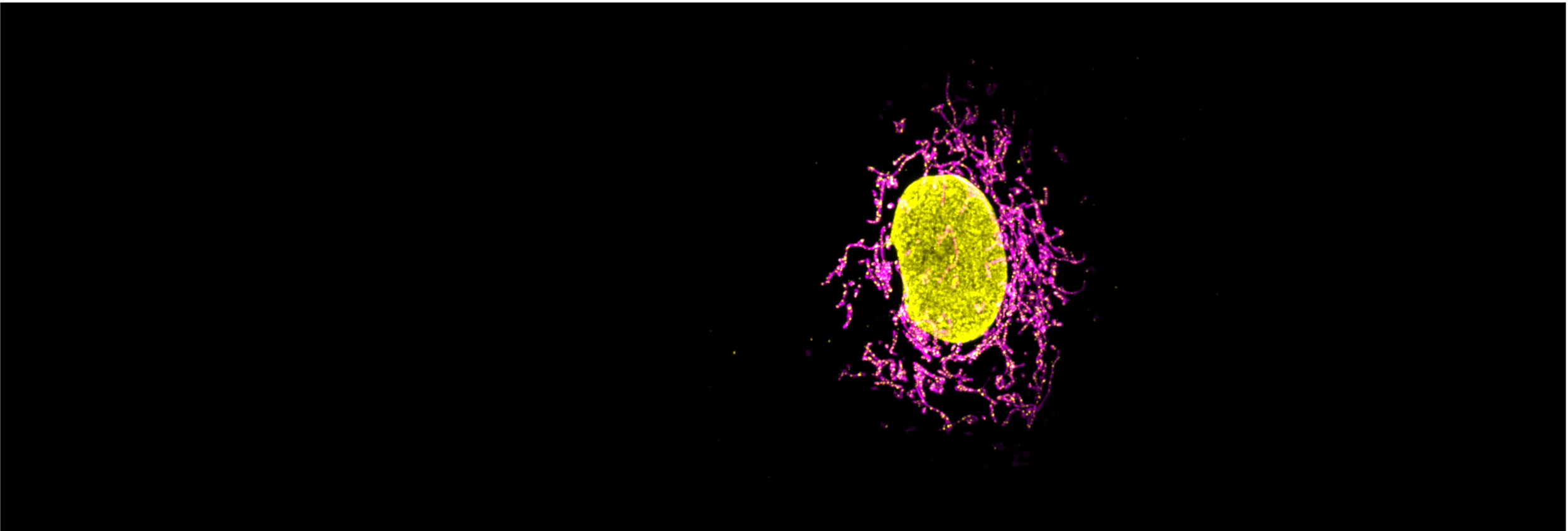
Our research focuses on multiple aspects of mitochondrial plasticity and the functional implications of their dynamic behaviours in human health and disease. As we uncover new molecular mechanisms that regulate mitochondrial dynamics we are led into the worlds of cancer, neurodegeneration, metabolism, and more.
-
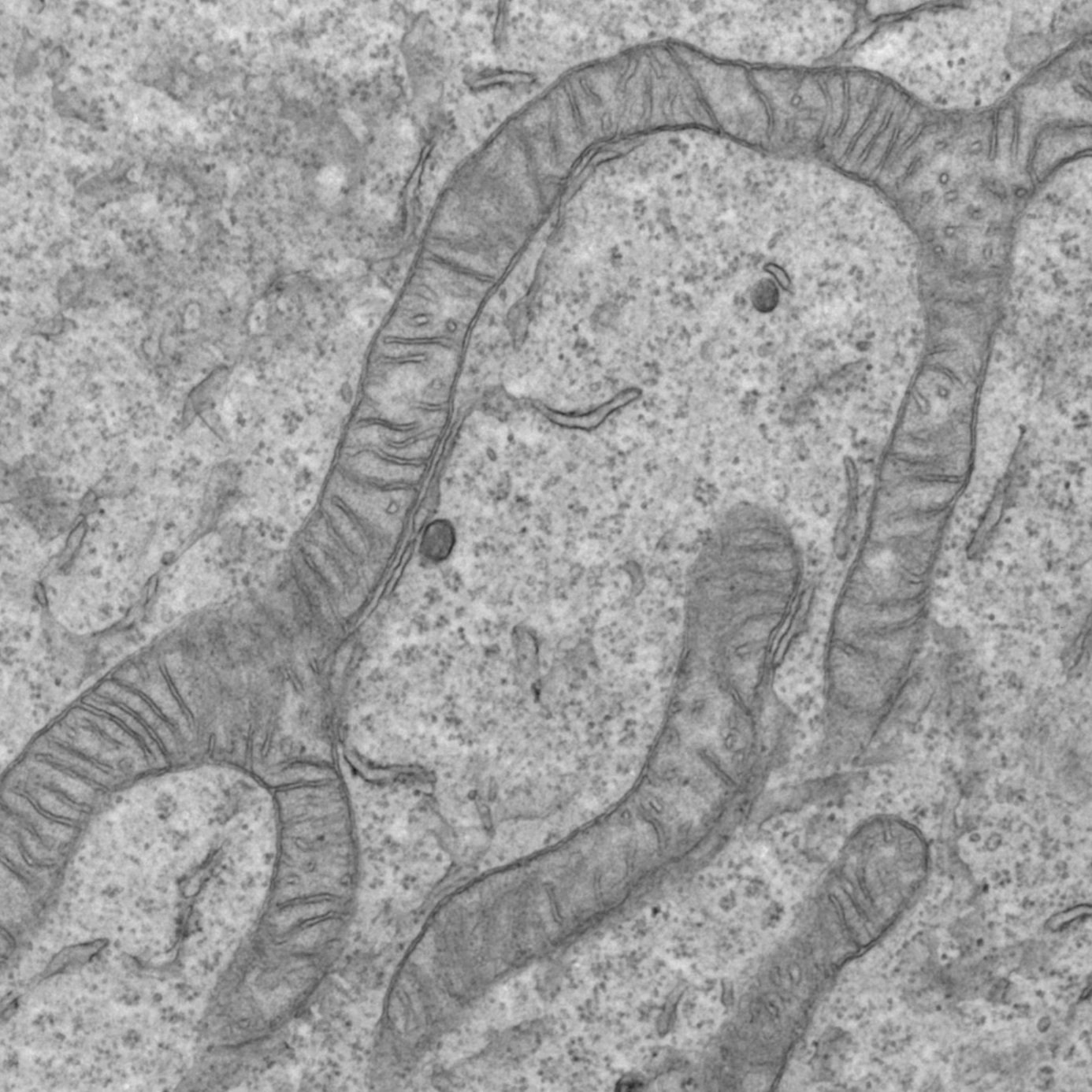
Mechanisms of mitochondrial fusion & fission
-

Characterization of mitochondrial-derived vesicles
-

The role of SUMOylation in mitochondrial & cellular signaling
-

Mitochondria and immunity in Parkinsons Disease
-

Mechanisms of mitochondrial iron transport and homeostasis
-
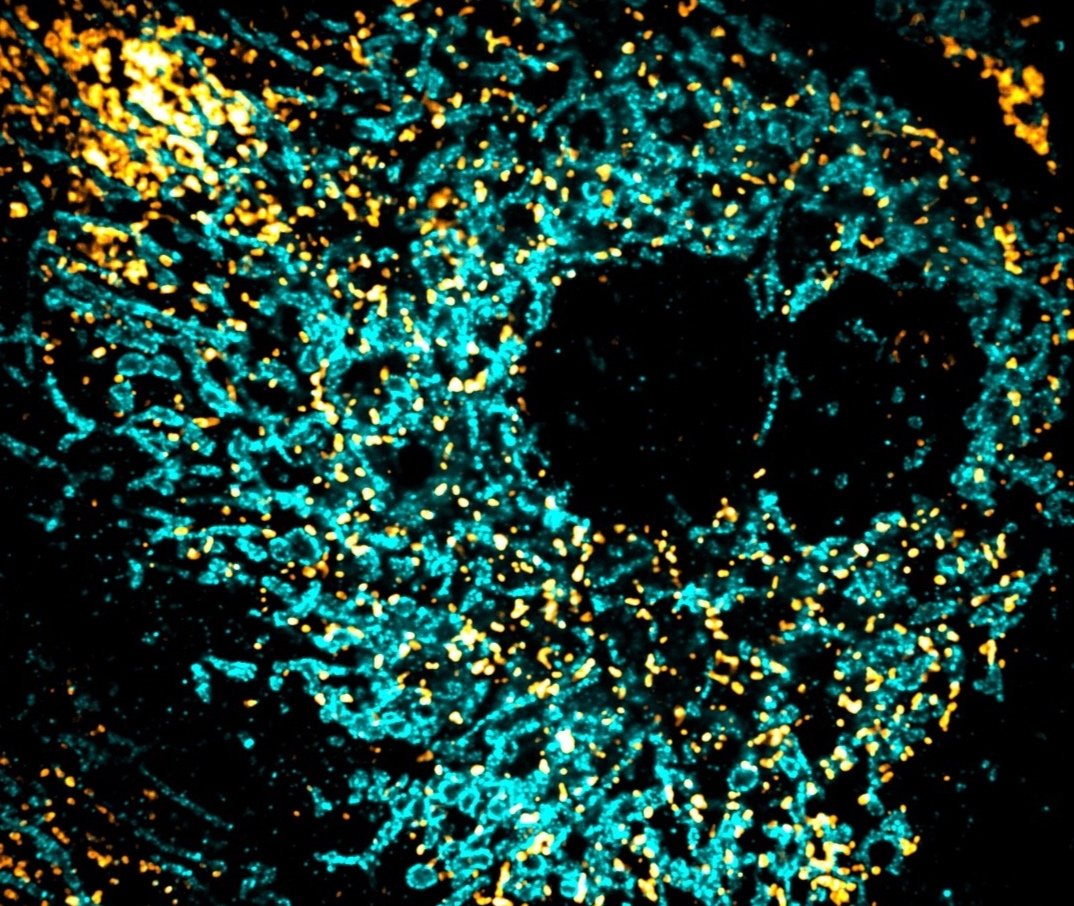
Understanding peroxisome biogenesis, homeostasis & function










